Interventional Cardiology
FASTER & SAFER HEMOSTASIS
Standardize, simplify and minimize post procedure care and maintenance from cath lab procedures with StatSeal topical hemostatic products. The products rapidly form an occlusive seal over access sites to stop the flow of blood and exudates; the seal strengthens over time and with pressure. StatSeal products work independently of the clotting cascade to seal access sites, while accelerating hemostasis and minimizing complications.1-5
THE STATSEAL SOLUTION
Comprised of a hydrophilic polymer and potassium ferrate, StatSeal products are available in both powder and disc (compressed powder) form to suit a wide variety of clinical applications. From sheath removals after diagnostic and interventional procedures, to bleeding control after closure device deployment, integrating StatSeal products into cath lab protocols has been found to result in significant clinical, economical and operational efficiencies.1-5
- Significantly accelerates hemostasis1-5
- • Reduces hold times
- • Works regardless of anticoagulation levels
- Minimizes post procedure care1-5
- • Reduces complication rates
- • Facilitates same-day discharge
- Improves cath lab efficiency1-5
- • Reduces clinician burden, time and costs
- • Increases patient throughput
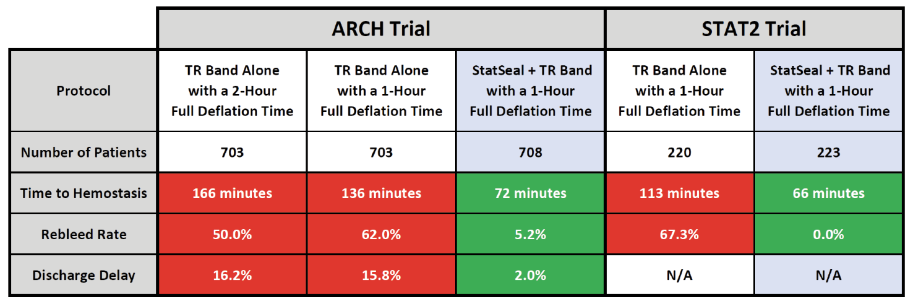
RECENT CLINICAL DATA
The very positive results from the ARCH Trial mirrored the results from the STAT2 Trial. These two synergistic randomized controlled trials cover two continents, four hospitals, and over 2,500 patients. As shown in the table below, the results clearly indicate significant clinical and operational benefits with StatSeal 1-hour protocols. These trials also demonstrate what happens without StatSeal use; more than 60% of cases rebleed after 1 hour and 50% still rebleed after 2 hours.2,3
PRODUCTS FOR CATH LAB PROCEDURES
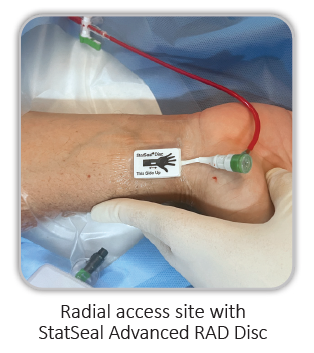
Radial
StatSeal® Advanced RAD Disc, in conjunction with manual pressure or any radial hemostasis band, can help standardize, simplify and shorten the recovery process to under an hour, while minimizing complications.2-5

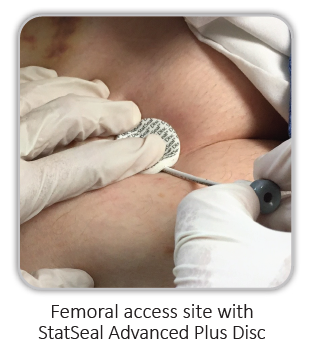
Femoral
As an adjunct to manual pressure, StatSeal® Advanced Plus Disc helps control external bleeding from sheath removals and oozing after closure device deployment, while minimizing hematoma risk.1

Electrophysiology
StatSeal Advanced Plus Disc helps achieve fast, consistent hemostasis on multiple puncture sites, drastically shortening recovery time without increasing complications, even on patients prone to bleeding.1

HOW DOES IT WORK?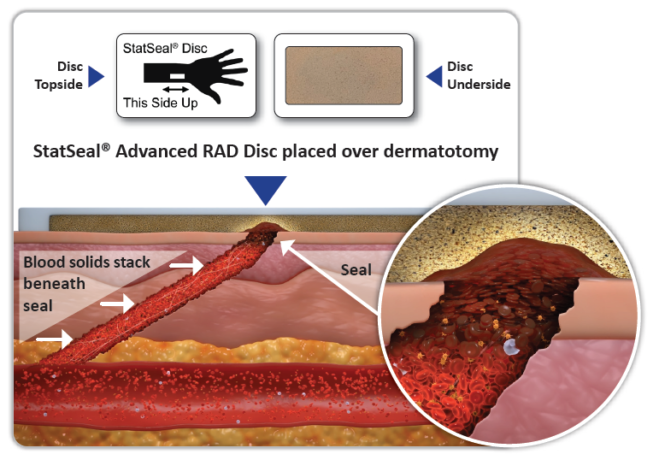
As an adjunct to pressure, StatSeal products have a two-step mechanism of action that occurs simultaneously to instantly form a low pH, occlusive seal.
- • The hydrophilic polymer rapidly dehydrates the blood and absorbs exudate, stacking up desiccated blood solids beneath to form a seal.
- • The potassium ferrate binds the blood solids and proteins together, adhering the seal to the wound to stop bleeding and oozing.
- Beneath the seal, the pH is neutral and the blood solids and proteins continue to stack. Above the seal, the hydrophilic polymer exchanges protons for cations, resulting in desiccation and a pH of ~2. The distal tract seal helps promote stasis at the arteriotomy.6
References: [1] Peralta R, Sharma A, Srinivasan N, et al. Stat Seal Groin Closure After AF Ablation to Allow Rapid Same Day Discharge. AF Healthcare Pioneer Report. Jun 2023: 19-20. [2] Proscia C, et al. Assessment of Radial Artery Complications Whilst Achieving Rapid Haemostasis -ARCH Trial. Presentation at: EuroPCR; May, 2022; Paris, France. [3] Safirstein JG, Tehrani DM, Schussler JM, et al. Radial Hemostasis Is Facilitated With a Potassium Ferrate Hemostatic Patch: The STAT2 Trial. JACC Cardiovasc Interv. 2022 Apr 25;15(8):810-819. [4] Galusko V, Protty M, Bharucha A, et al. TCT-781 The Quest for a Radial Lounge: StatSeal Reduces Transradial Coronary Angiography Turn-Around Time and Cost. JACC Suppl B. 2019; 24(13)B765. [5] Khuddus M, Massaro J, Klass D, at al. TCT-792 Meta-Analysis of Radial Hemostasis Trials Using Patent Hemostasis and a Potassium Ferrate Hemostatic Disc. JACC Suppl B. 2019; 24(13)B776. [6] Biolife, LLC, 510(k) K080210, Section 18.3.